| Availability: | |
|---|---|
Product Structure:

Reagent Storage Area
The interior maintains a stable temperature of 2 - 8℃ for storing bagged culture media, preventing performance degradation caused by repeated pre - warming of reagents during manual cultivation.
Human - Machine Interaction Area
It allows for the editing and running of the cultivation process, displays and saves environmental data during cultivation, and the operation interface is simple and easy to use.
Exosome Culture Area
The incubator provides a suitable cell culture environment, maintaining the set temperature and carbon dioxide concentration inside to achieve automated cell cultivation. The swing platform is fixed inside the incubator and swings according to the programmed settings, accelerating cell growth and facilitating exosome harvesting.
Product Advantages:
Automation
Equipped with a disposable fully - enclosed pipeline system with pre - packaged flake - shaped microcarriers, which can be used immediately upon retrieval. The entire process from cell culture to exosome production is automated. This reduces requirements for the external environment and saves costs.
Scalability
The system automatically records all operation steps in the whole process, providing more precise and consistent culture conditions. It offers a more intelligent and scalable culture solution for exosome production in small - and medium - sized systems.
Standardization
It provides a mature and complete production process with standardized production procedures. A set of standardized production processes can be quickly replicated in multiple laboratories of users, helping users rapidly establish their own product systems.
Core Technology 1: (Bagged Liquid Medium for Mesenchymal Stem Cells)
The exosome culture workstation enables the automated production of exosomes, relying on the bagged liquid medium. During the manufacturing process of this product, it is directly filled into a sterile bag. During use, it enters the cell culture bag through a fully enclosed pipeline system, without any contact with the outside environment throughout the process, ensuring that the medium remains sterile at all times. This packaging method effectively avoids external contamination and guarantees the safety of exosome production.
The bagged culture medium is packaged with multi - layer co - extruded film. This film has the advantages of high barrier properties, strong functionality, high strength, and no pollution, and it also exhibits excellent biocompatibility.
Core Technology 2: (Pre - packaged Sterile Flake - shaped Microcarriers)
The flake - shaped carrier is mainly made of medical - grade polymer polyester fiber (PET). Through electrostatic ultrasonic spinning technology, it is thermally bonded and processed into a non - woven fabric substrate with a porous structure. After further processes such as welding, shaping, cleaning, and surface modification, it is prepared into a flake - shaped solid growth - supporting matrix for adherent - dependent cell culture.
As the main material of the flake - shaped carrier, the 3D reticular three - dimensional structure fabric formed by PET has characteristics such as good acid - and alkali - resistance, heat - resistance, non - toxicity, and non - biodegradability. It has moderate stiffness and good flexibility, and can well tolerate high fluid stress. There are no additives during the processing, which is non - toxic and harmless to cell growth and meets the requirements of pharmaceutical manufacturing.
The flake - shaped carrier is a highly efficient micro - carrier specifically designed for adherent culture of mammalian cells. It has a single - layer thickness of 0.44 mm and a pore size of approximately 15 μm. It can provide sufficient surface area for cell growth, enabling cells to reproduce and grow in a three - dimensional structural space. At the same time, it maintains good exchange of various nutrients and reduces the accumulation of harmful metabolites, providing a favorable micro - environment for cell growth and achieving high - density cell culture.
Since the carrier has a certain cell - retaining effect, when obtaining the cell harvest solution, there is no need to use cell - retaining devices such as rotary filters or sedimentation columns. This reduces the content of cells and DNA in the harvest solution. Compared with other culture systems, it is more conducive to the downstream purification of exosome products.
Core Technology 3: (Fully Enclosed Single - use Pipeline System)

FEP Membrane Material
FEP is short for perfluoroethylene propylene copolymer. It is a 100% inert material, a single - layer, high - purity substance. No additives or other biological source materials are added during the processing, showing excellent biocompatibility.
FEP has good oxygen permeability and water vapor barrier properties, which can meet the gas exchange required during cell growth. Under the same culture volume, a higher number of cells can be obtained.
FEP has a light transmittance of over 95%, allowing for a very intuitive observation of the cells inside the bag. This enables a better assessment of the cell growth state and ensures the safety of the cells.
Silicone Tube
Medical - grade silicone tubes are used. The material has passed relevant biocompatibility tests and exhibits good chemical stability, meeting the requirements of high - temperature sterilization and irradiation sterilization.
Injection - molded Parts
All injection - molded parts are made of medical - grade raw materials, which have good biocompatibility, providing good connection stability and safety for the entire system.
Experimental Data
1、Exosome concentration, particle size
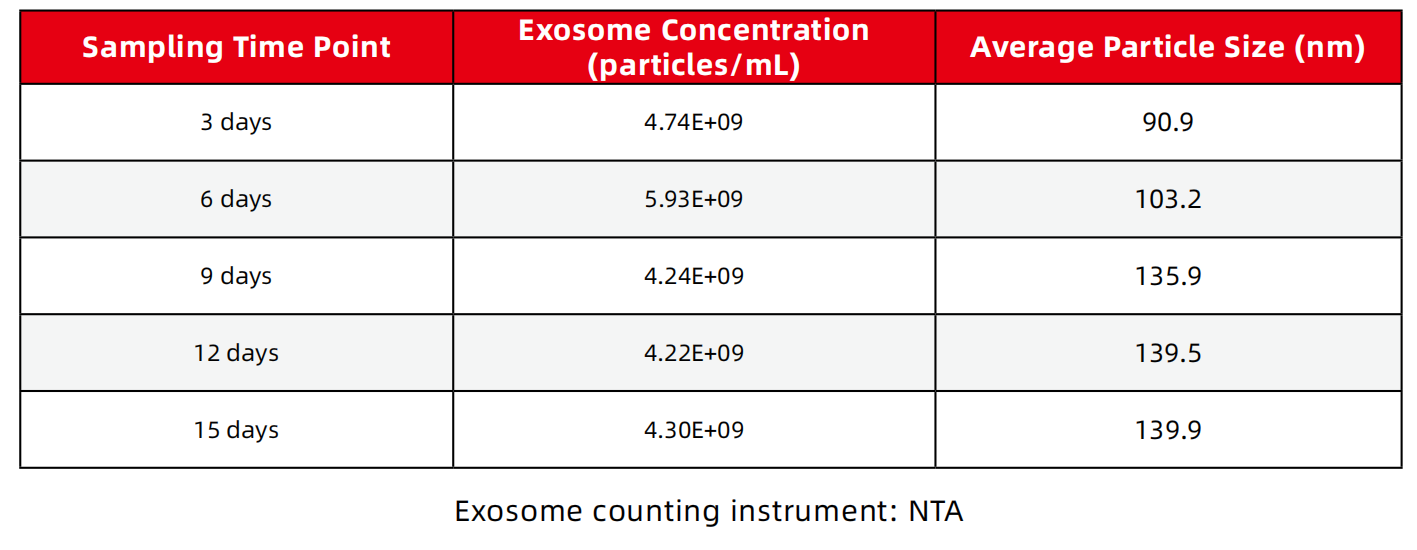
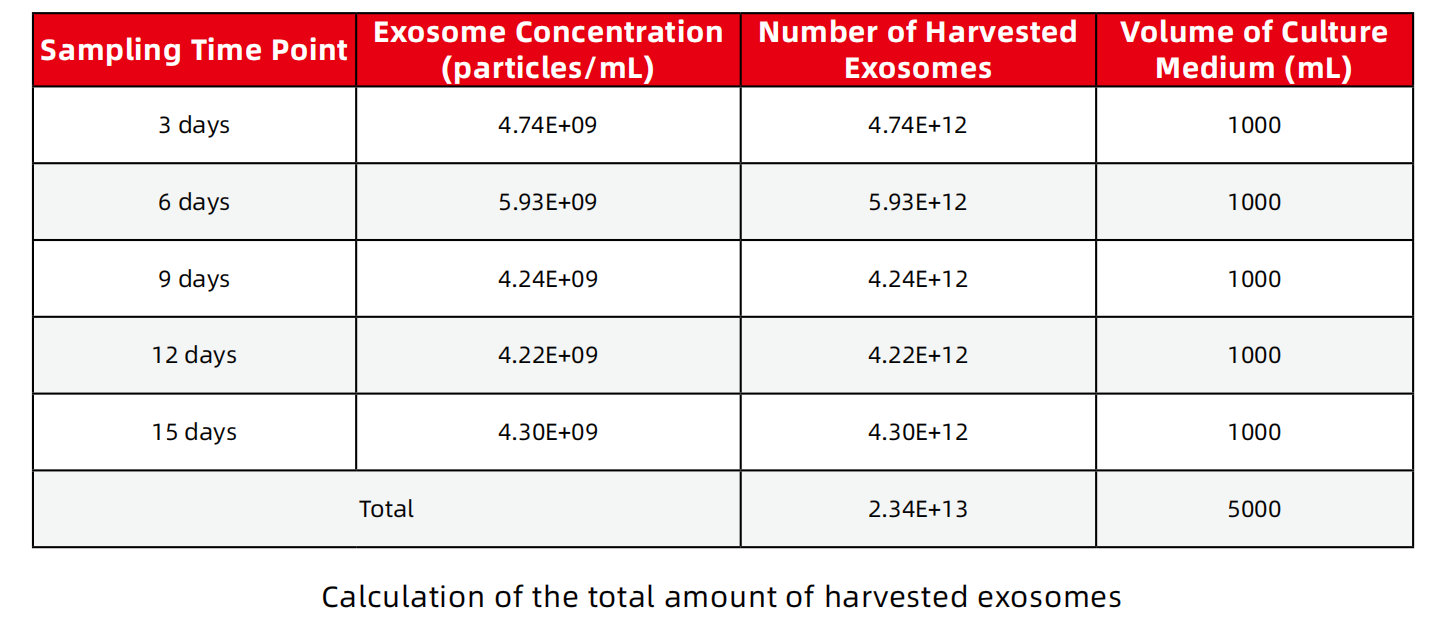
2、The total amount of harvested exosomes
A cultivation cycle is 15 days, requiring 5L of culture medium. There is a cycle every 3 days, and 1L of supernatant can be harvested. The total number of harvested exosomes is 2.34E+13.
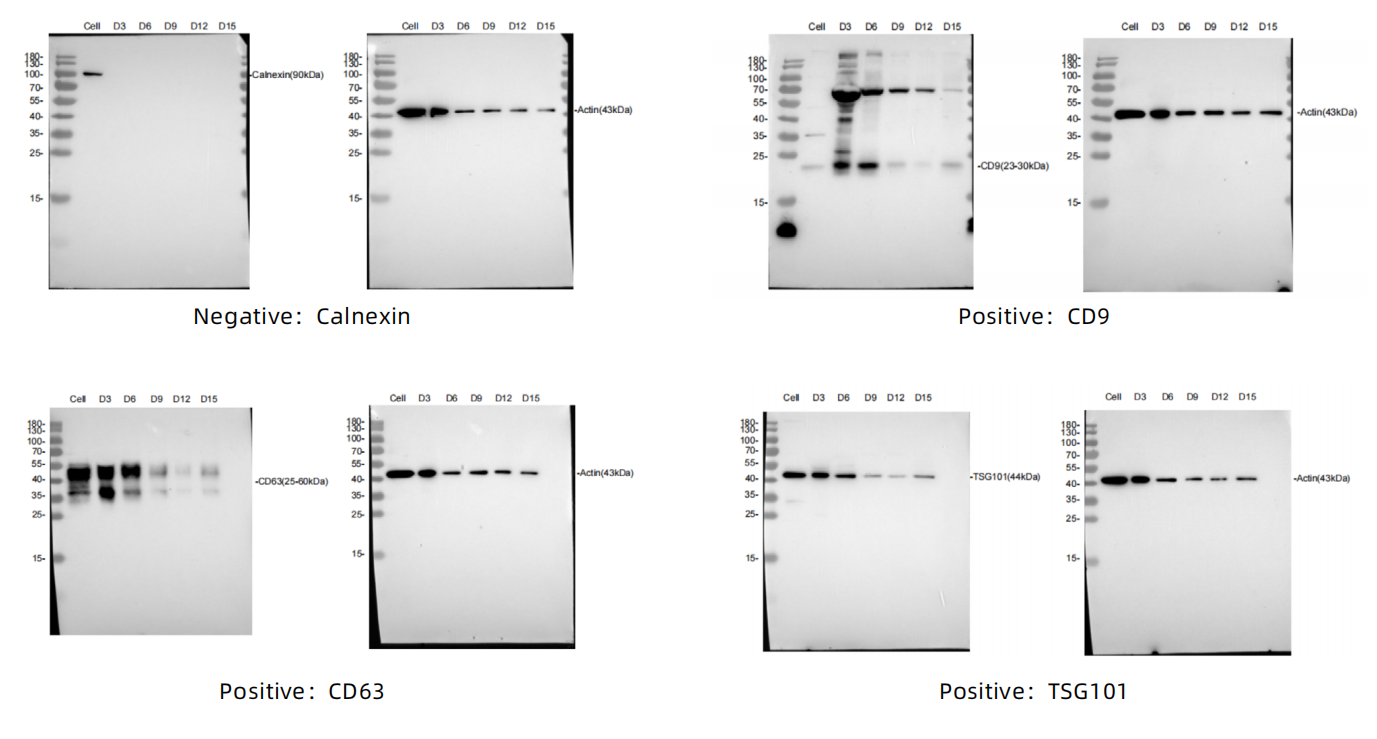
3、Exosome - marker proteins
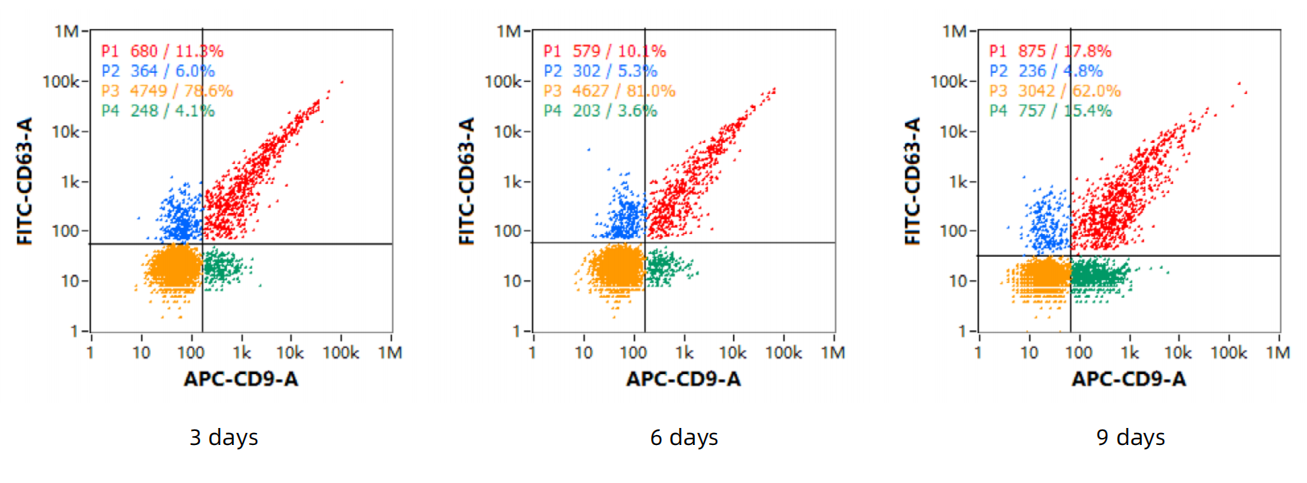
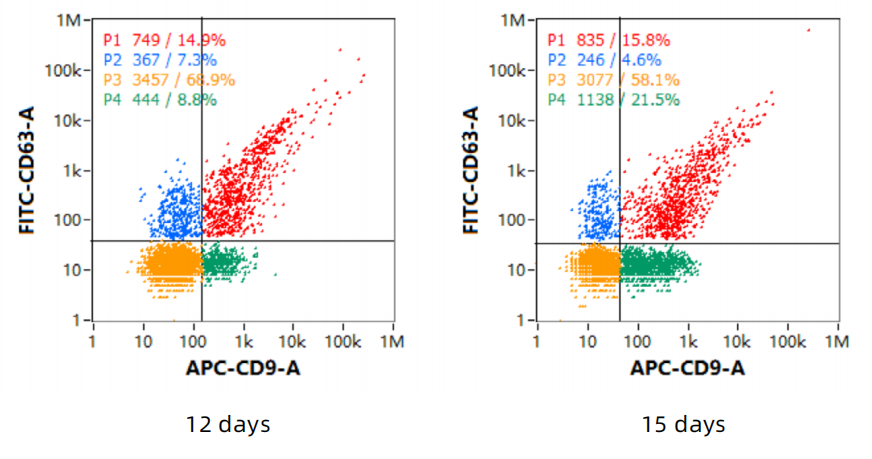
4、Detect the protein markers by Western - Blot (WB)
Negative: Calnexin
Positive: CD9, TSG101, CD63
Product Structure:

Reagent Storage Area
The interior maintains a stable temperature of 2 - 8℃ for storing bagged culture media, preventing performance degradation caused by repeated pre - warming of reagents during manual cultivation.
Human - Machine Interaction Area
It allows for the editing and running of the cultivation process, displays and saves environmental data during cultivation, and the operation interface is simple and easy to use.
Exosome Culture Area
The incubator provides a suitable cell culture environment, maintaining the set temperature and carbon dioxide concentration inside to achieve automated cell cultivation. The swing platform is fixed inside the incubator and swings according to the programmed settings, accelerating cell growth and facilitating exosome harvesting.
Product Advantages:
Automation
Equipped with a disposable fully - enclosed pipeline system with pre - packaged flake - shaped microcarriers, which can be used immediately upon retrieval. The entire process from cell culture to exosome production is automated. This reduces requirements for the external environment and saves costs.
Scalability
The system automatically records all operation steps in the whole process, providing more precise and consistent culture conditions. It offers a more intelligent and scalable culture solution for exosome production in small - and medium - sized systems.
Standardization
It provides a mature and complete production process with standardized production procedures. A set of standardized production processes can be quickly replicated in multiple laboratories of users, helping users rapidly establish their own product systems.
Core Technology 1: (Bagged Liquid Medium for Mesenchymal Stem Cells)
The exosome culture workstation enables the automated production of exosomes, relying on the bagged liquid medium. During the manufacturing process of this product, it is directly filled into a sterile bag. During use, it enters the cell culture bag through a fully enclosed pipeline system, without any contact with the outside environment throughout the process, ensuring that the medium remains sterile at all times. This packaging method effectively avoids external contamination and guarantees the safety of exosome production.
The bagged culture medium is packaged with multi - layer co - extruded film. This film has the advantages of high barrier properties, strong functionality, high strength, and no pollution, and it also exhibits excellent biocompatibility.
Core Technology 2: (Pre - packaged Sterile Flake - shaped Microcarriers)
The flake - shaped carrier is mainly made of medical - grade polymer polyester fiber (PET). Through electrostatic ultrasonic spinning technology, it is thermally bonded and processed into a non - woven fabric substrate with a porous structure. After further processes such as welding, shaping, cleaning, and surface modification, it is prepared into a flake - shaped solid growth - supporting matrix for adherent - dependent cell culture.
As the main material of the flake - shaped carrier, the 3D reticular three - dimensional structure fabric formed by PET has characteristics such as good acid - and alkali - resistance, heat - resistance, non - toxicity, and non - biodegradability. It has moderate stiffness and good flexibility, and can well tolerate high fluid stress. There are no additives during the processing, which is non - toxic and harmless to cell growth and meets the requirements of pharmaceutical manufacturing.
The flake - shaped carrier is a highly efficient micro - carrier specifically designed for adherent culture of mammalian cells. It has a single - layer thickness of 0.44 mm and a pore size of approximately 15 μm. It can provide sufficient surface area for cell growth, enabling cells to reproduce and grow in a three - dimensional structural space. At the same time, it maintains good exchange of various nutrients and reduces the accumulation of harmful metabolites, providing a favorable micro - environment for cell growth and achieving high - density cell culture.
Since the carrier has a certain cell - retaining effect, when obtaining the cell harvest solution, there is no need to use cell - retaining devices such as rotary filters or sedimentation columns. This reduces the content of cells and DNA in the harvest solution. Compared with other culture systems, it is more conducive to the downstream purification of exosome products.
Core Technology 3: (Fully Enclosed Single - use Pipeline System)

FEP Membrane Material
FEP is short for perfluoroethylene propylene copolymer. It is a 100% inert material, a single - layer, high - purity substance. No additives or other biological source materials are added during the processing, showing excellent biocompatibility.
FEP has good oxygen permeability and water vapor barrier properties, which can meet the gas exchange required during cell growth. Under the same culture volume, a higher number of cells can be obtained.
FEP has a light transmittance of over 95%, allowing for a very intuitive observation of the cells inside the bag. This enables a better assessment of the cell growth state and ensures the safety of the cells.
Silicone Tube
Medical - grade silicone tubes are used. The material has passed relevant biocompatibility tests and exhibits good chemical stability, meeting the requirements of high - temperature sterilization and irradiation sterilization.
Injection - molded Parts
All injection - molded parts are made of medical - grade raw materials, which have good biocompatibility, providing good connection stability and safety for the entire system.
Experimental Data
1、Exosome concentration, particle size

2、The total amount of harvested exosomes
A cultivation cycle is 15 days, requiring 5L of culture medium. There is a cycle every 3 days, and 1L of supernatant can be harvested. The total number of harvested exosomes is 2.34E+13.

3、Exosome - marker proteins


4、Detect the protein markers by Western - Blot (WB)
Negative: Calnexin
Positive: CD9, TSG101, CD63
